A Cluster Randomized Clinical Trial of the NASG at the Primary Health Care Level before Transport to Referral Hospitals was a Multi-Center Center Study conducted in Zambia and Zimbabwe

NASG Dissemination Meeting: First Lady urges use of NASG

ZambiaFirst Lady, Dr. Christine Kaseba with Dr. Suellen Miller and traditional Zambian dancer at the meeting.
The NASG Zambia dissemination meeting was held in Lusaka on April 24, 2013 and included attendance by local government officials, including the First Lady Dr. Christine Kaseba. She urges the use of the Non-pneumatic Anti-Shock Garment as a tool to reduce obstetric hemorrhage. Read the Lusaka Voice article here.
Project Background

(L to R Zambia NASG staff Rachel Sumotwe, First Lady Dr Kaseba, NASG Project PI Dr. Suellen Miller, NASG Project Director Elizabeth Butrick
The Zimbabwe NASG dissemination meeting was held in Harare on May 3, 2013. In attendance were high-level government officials including the Health & Child Welfare Secretary Dr. Gerald Gwinji. The meeting was well attended by local project staff and enthusiastic health providers eager for news of the trial results. Read the local press coverage here. The meeting was highlighted by an official NASG donation and a moving testimony of an NASG survivor, Tatenda Mugariri (shown at left). Read Tatenda’s story here.
In Zambia, only 44% of pregnant women deliver in a health care facility. The maternal mortality ratio is 603 (603 women die for every 100,000 live births) and the lifetime risk of maternal death is 1 in 27 (UNICEF, 2009). In Zimbabwe, 69% of women have a skilled attendant at birth but the maternal mortality ratio is still very high at 624 (624 deaths for every 100,000 live births) and a lifetime risk of maternal death of 1 in 43 (UNICEF, 2005).
In 2007 a large cluster-randomized controlled trial (RCT) was initiated in Zambia and Zimbabwe. This was undertaken to to explore the crucial question of whether the NASG, applied earlier by midwives at primary health care centers, will save more mothers’ lives than application later only at higher-level facilities. This was our first study to see whether early application of the NASG results in even better outcomes than those we have seen when applied only at the higher-level facilities.
 We had two sites in Zambia, one in the capital, Lusaka, and one in the Copperbelt province. In Lusaka we worked at the University Teaching Hospital and 12 clinics that refer to this hospital. In the Copperbelt we worked at Kitwe Central Hospital and 7 clinics that refer to this hospital, and Ndola Central hospital and 7 clinics that refer to this hospital. Our personnel in Zambia included local Principal Investigators Dr. Christine Kaseba and Dr. Gricelia Mkumba. The Lusaka Study Coordinator was Nurse-Midwife Rhoda Amafumba, and clinic and hospital Coordinators Ameldah Kabwe, Joyce Mwami, Rachael Simutowe, Hope Naganyika, Mary Sieta and Finely Sovi.
We had two sites in Zambia, one in the capital, Lusaka, and one in the Copperbelt province. In Lusaka we worked at the University Teaching Hospital and 12 clinics that refer to this hospital. In the Copperbelt we worked at Kitwe Central Hospital and 7 clinics that refer to this hospital, and Ndola Central hospital and 7 clinics that refer to this hospital. Our personnel in Zambia included local Principal Investigators Dr. Christine Kaseba and Dr. Gricelia Mkumba. The Lusaka Study Coordinator was Nurse-Midwife Rhoda Amafumba, and clinic and hospital Coordinators Ameldah Kabwe, Joyce Mwami, Rachael Simutowe, Hope Naganyika, Mary Sieta and Finely Sovi.
In Zimbabwe we worked with the UZ-UCSF Collaborative Research Programme at Harare Central Hospital, Parienyatwa Hospital and 12 clinics that refer patients to these hospitals. Our personnel in Zimbabwe included local Principal Investigators Dr. Thulani Magwali and Dr. Michael Chirenje, and Harare Study Coordinator Violet Mambo, and the dedicated study midwives.
 Randomized Cluster Trials represent the gold standard in research; in our study of 880 women, the NASG intervention resulted in a 54% reduction in deaths and/or morbidities, a category we called “Extreme Adverse Outcomes” (odds ratio, OR 0.46; 95% CI 0.13–1.62) and a non-significant 46% reduced odds of maternal mortality (OR 0.54; 95% CI 0.14–2.05), these findings are clinically important, but not statistically significant. . This study demonstrated a statically significant 25% faster shock recovery time for the women who received the NASG earlier as compared to those who did not receive NASG until the RH. A complete description of our study methods and findings can be read in Non-Pneumatic Anti-Shock Garment (NASG), a First-Aid Device to Decrease Maternal Mortality from Obstetric Hemorrhage: A Cluster Randomized Trial.
Randomized Cluster Trials represent the gold standard in research; in our study of 880 women, the NASG intervention resulted in a 54% reduction in deaths and/or morbidities, a category we called “Extreme Adverse Outcomes” (odds ratio, OR 0.46; 95% CI 0.13–1.62) and a non-significant 46% reduced odds of maternal mortality (OR 0.54; 95% CI 0.14–2.05), these findings are clinically important, but not statistically significant. . This study demonstrated a statically significant 25% faster shock recovery time for the women who received the NASG earlier as compared to those who did not receive NASG until the RH. A complete description of our study methods and findings can be read in Non-Pneumatic Anti-Shock Garment (NASG), a First-Aid Device to Decrease Maternal Mortality from Obstetric Hemorrhage: A Cluster Randomized Trial.
The outcomes of our research have resulted in the World Health Organization’s inclusion of the NASG on its list of essential devices, which has enabled governments and NGOs to start buying and implementing the NASG on a much larger scale.
Funding for this study was provided by the National Institutes for Child Health and Development (NICHD) and the Bill and Melinda Gates Foundation.
References
The Non-pneumatic Anti-Shock Garment: How Applier Strength and Body Mass Index Affect External Abdominal Pressure. Stenson, A; Lester, F; Meyer, C; Morris, JL; Vargas, J; Miller, S (2011) Open Womens Health J; 5, 33-37.
Impact of the Non-pneumatic Antishock Garment on pelvic blood flow in healthy postpartum women. Lester, F.; Stenson, A.; Meyer, C.; Morris, J.; Vargas, J.; Miller, S. Am J Obstet Gynecol, 2011; 204(5):409e1-e5
Nigeria
The most populous country in Africa, Nigeria, also has one of the highest maternal mortality ratios at 608 maternal deaths per every 100,000 live births.
Safe Motherhood began working in Nigeria in 2003, with funding from the John D. and Catherine T. MacArthur Foundation. Over the next 4 years our study grew to include 12 hospitals, covering 4 states in Northern and Southern Nigeria. We carried out a pre-intervention, intervention study to compare how well women treated with the NASG did compared to women in the same hospitals on whom the NASG was not used.
Over the course of four years we collected observations on 756 women; 182 in the pre-intervention group and 574 women in the NASG group. Fewer women were included in the pre-intervention group because once the providers in Nigeria began using the NASG they immediately noticed the difference in outcomes and stakeholders wanted to bring this life-saving technology to women as quickly as possible.
When we compared the outcomes of the two groups, the NASG group fared much better, in spite of starting the study in worse condition. They lost less additional blood after entry to the study, were less likely to develop a debilitating complication (morbidity), and were less likely to die than those in the pre-intervention group. These results were statistically significant and can be read about on our scientific publications page.
We are indebted to the committed doctors, nurses and midwives of Nigeria, especially the collaborating Principal Investigators, Professor Oladosu Ojengbede, Prof Morhason Bello, Prof Khadija Galandanci, Dr. David Nsima, Dr. Mohammed Awaal for their hard work in documenting the successes of the NASG. For years to come women will owe their lives to these pioneering researchers!
Egypt
Egypt has made great progress in reducing deaths from pregnancy and childbirth. In 2007 the maternal mortality ratio had been reduced to 46 per 100,000, but preventable and treatable obstetric hemorrhage continues to be the leading cause of maternal deaths.
Dr. Suellen Miller and Egyptian colleagues part of the USAID/JSI maternal health project under the guidance of Dr. Reginald Gipson and Dr. Sabry Hamza, embarked on the NASG Egypt pilot study in 4 hospitals in Egypt through the contributions and support of the John D. and Catherine T. MacArthur Foundation in 2004. The main purpose of the study was to find out whether applying the NASG to women experiencing obstetric hemorrhage would reduce blood loss and reverse shock.
The first study conducted in Egypt involved 364 women and showed that women in the NASG group had 50% less blood loss and 69% fewer deaths and/or morbidities, a category we called “Extreme Adverse Outcomes” (EAOs), as compared to the pre-intervention group.
A subsequent comparative study was conducted at two referral facilities in Cairo and Assiut. The local Principal investigators were Mohammad Mourad-Youssif and Mohamed Fathallah. There were 432 women in the pre-intervention group and 588 women in the NASG group and showed that women in the NASG group had a 54% lower risk of dying, 78% lower risk of acquiring a severe complication involving organ failure and a 68% lower risk of having an “EAO”. Further, women in the NASG group also had 33% less measured blood loss and 42% less blood loss in surgical cases. Link to publications about Egypt (2) 2009 and forward, should include combined with Nigeria
These results show the NASG is promising for management of women with obstetric hemorrhage and that it is effective and useful in developing countries that have a well developed health care system and lower maternal mortality rates, serving to compensate for the delays and other problems that even busy urban hospitals may have.
These studies were funded by the MacArthur Foundation with in-kind donations from USAID/JSI.
USA
Mechanisms of Action Studies
 We continue to carry out small studies in the U.S. in order to further investigate the mechanisms of action of the NASG. These findings will help us improve our training, use and understanding of this important device.
We continue to carry out small studies in the U.S. in order to further investigate the mechanisms of action of the NASG. These findings will help us improve our training, use and understanding of this important device.
So far this has involved a hemodynamic study of 10 women post-delivery at San Francisco General Hospital led by Dr. Juan Vargas and Dr. Felicia Lester; a Manometer Study of 10 women at UCSF Medical Center led by Dr. Felicia Lester and Carinne Meyer, and a second manometer study on the effect of BMI and strength of application of the garment on 10 women led by Dr.Amy Stenson, also at UCSF Medical Center.
The NASG is also included in CMQCC’s (California Maternal Quality Care Collaborative) Hemorrhage Taskforce Toolkit. View this resource.
Case Series to Investigate the Hemodynamic Impact of the Non-Pneumatic Anti-Shock Garment (NASG) in Post-Partum Women by Felicia Lester, MD, MS, MPH; Suellen Miller, CNM, PhD; Juan Vargas, MD. Click on the image to see this poster:
Mexico
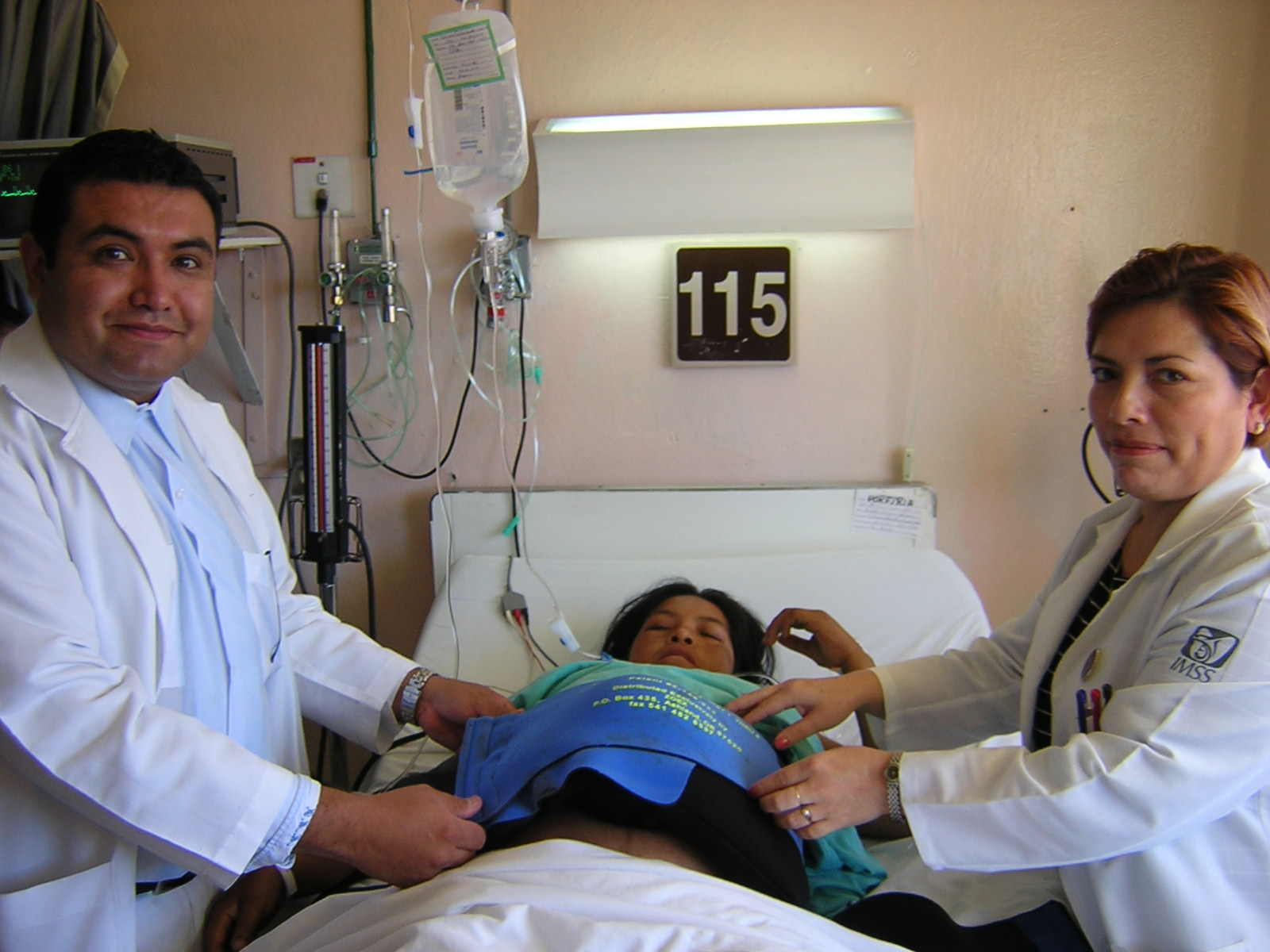 Between 2004 and 2006, Population Council conducted research in rural areas of Puebla and Oaxaxa in Mexico to determine whether the NASG (or “TAN” – Traje anti-shocque no pnumatico) would be considered to be an acceptable first-aid device by staff in primary health care centers. This research identified methodological and practical ways to ensure that the NASG is accepted into rural health care systems.
Between 2004 and 2006, Population Council conducted research in rural areas of Puebla and Oaxaxa in Mexico to determine whether the NASG (or “TAN” – Traje anti-shocque no pnumatico) would be considered to be an acceptable first-aid device by staff in primary health care centers. This research identified methodological and practical ways to ensure that the NASG is accepted into rural health care systems.
 Read about 4 trajectories of acceptance/rejection identified by work in Mexico. A manuscript on the results has been published by ‘Health care for Women International’ and is available on our scientific publication page or here titled: Acceptance of a New Technology for Management of Obstetric Hemorrhage: A Qualitative Study from Mexico.
Read about 4 trajectories of acceptance/rejection identified by work in Mexico. A manuscript on the results has been published by ‘Health care for Women International’ and is available on our scientific publication page or here titled: Acceptance of a New Technology for Management of Obstetric Hemorrhage: A Qualitative Study from Mexico.

Recent Comments